In the realm of industrial product development and manufacture, the demand for accuracy and repeatability in testing processes is non-negotiable. The significance of advanced testing software in attaining these business goals cannot be underestimated, as it can help ensure that products and materials adhere to relevant quality standards.
This article delves into the critical requirements for physical properties testing software in the industrial and medical sectors, with a primary focus on the “artificial intelligence” that should interface hand-in-hand with human users, to deliver “real intelligence” to quality assurance testing.
In the ever-evolving landscape of manufacturing, consistency in quality is one of the bedrocks upon which manufacturer’s reputations are built—and sustained. Physical properties testing software is the linchpin that enables the automating of testing procedures and in doing so, maximizes the accuracy, repeatability and reproducibility potential of results, significant metrics in ensuring product excellence.
Repeatability is the closeness of successive measurements when carried out by a single person or instrument on the same item, under the same conditions. Minimizing variability between the results of the identical test on the same sample is essential.
Reproducibility is the attribute where a different person or instrument can duplicate a test and obtain similar results. Not only does this refer to measurements taken from one day to the next, but in the context of a business with multiple national or global sites, this parameter is crucial in delivering a consistent level of quality to the end customer irrespective of geographical point of manufacture.
A software-controlled universal testing machine (UTM) is the choice of most manufacturing concerns to routinely perform mechanical strength tests for quality assurance. Investing in a single machine with the capability to perform multiple tests through software-controlled adjustments (and interchangeable fixtures) can be more cost-effective than purchasing several specialized testing machines.
Working together
Any level of process automation does require some human interaction and in a product testing workflow this is a vital element. The software partner of this [hu]man-machine team is responsible for those processes where it is best suited. This remit must encompass robust internal architecture and core functionality that all software must deliver:
- Compatibility (data exchange, communication)
- Flexibility (futureproofing, scalability, upgradeable)
- Security (confidentiality, accountability, integrity)
- Maintainability (standard IT methodologies and protocols)
Software taking the lead
The functionality of the software must facilitate the human user’s needs to achieve the business goals. Like a word processor must create documents, product testing software must determine and record whether the test specimen complies with legislative standards or meets functional performance targets.
Such a tool’s responsibility stretches beyond spell checking, with a brand’s reputation and the end customer’s safety being amongst the core aims of implementing such a quality practice.

Fully featured software both directs and protects the user within the specific purpose of their tasks. A user interface which enables seamless interaction with the hardware, software and network enables the human—of whatever skill level—to perform their role reliably. Software designed for a force or torque tester should ensure the correct machine is used, recommended grips are fitted, relevant accessory instrumentation is connected, and the appropriate test procedure is selected from an approved library. The objective should be a guided, error-free workflow, with the software taking responsibility for facilitating high levels of repeatability and reproducibility in the process.
Quick start guide
The value of human interaction in processes that involve automation and software lies in the complementary strengths of humans and technology, creating a synergy that enhances overall efficiency and effectiveness. The operator’s role in performing a test is vitally important, selecting the relevant test, loading the sample into the machine, and providing a check as to the success of the procedure. The user’s flexibility perfectly complements the rigid, repetitive task-performing strength of the software. Where these two “partners” closely interact is in the software providing guidance to the operator through the workflow. With the test routine established and programmed, an intuitive interface will preclude errors or safety compromises and control the machine in each operational step of the test.
Super for users

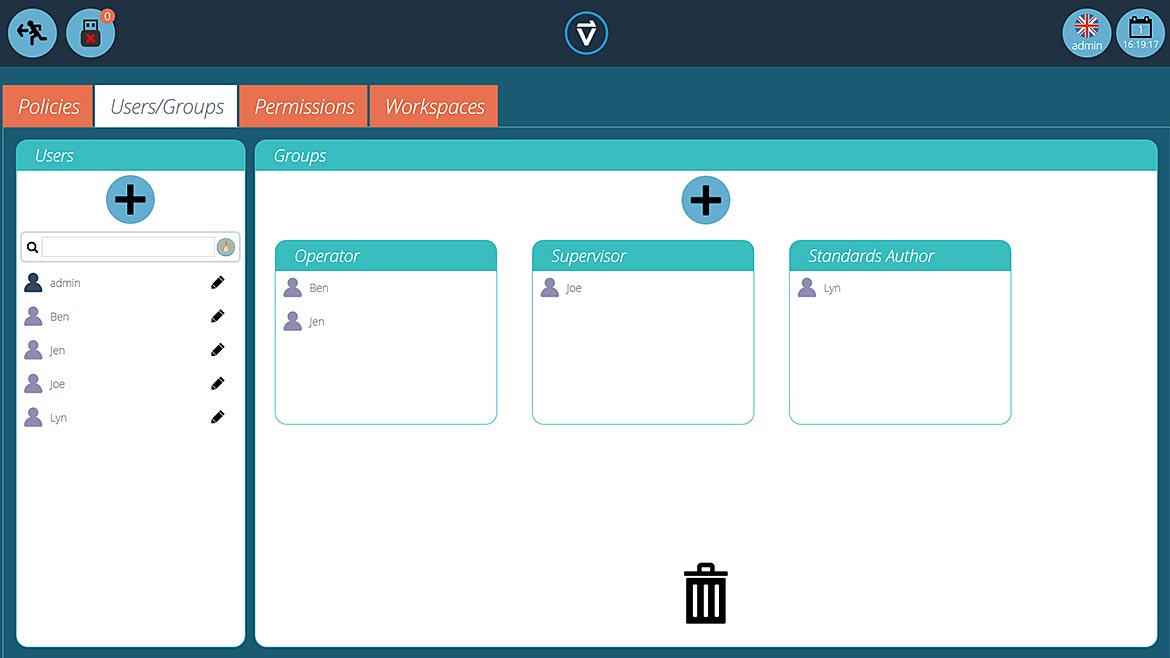
Role-based logins is one aspect of essential security measures that are now integral to industrial software applications. Permissions associated with operators, administrators and supervisors are all handled in the same way as general office computer systems. Networking and Active Directory functionality align the architecture with standard IT practices, bringing maintenance tasks into well-established processes.
Enabling security and accountability
Implementing software solutions internally, meeting your own business needs—and being validated by success in the market—is only part of the obligation in certain industries. The medical devices and pharmaceutical sector is highly regulated. Stringent physical testing standards exist for almost every functional aspect of a product intended for use in this domain, from verifying the glide force of a syringe plunger to measuring the opening torque of a drug container closure.
The United States Federal Drug Agency (FDA) mandates adherence to its code of regulations before it can grant approval for drugs and medical devices to be used. Compliance is non-negotiable. One essential aspect of regulatory requirement is FDA 21 CFR Part 11, which specifically addresses electronic records and signatures held as submissions to the FDA for regulatory approval. Since testing software contains electronic data which is often used as part of the regulatory approval process, the software solution must enable a robust and reliable audit trail.
Validating quality systems
FDA 21 CFR Part 11 relates specifically to electronic signatures and audit trail implementation through software. Industries to which it applies are also regulated by FDA 21 CFR Part 820 - Quality System Regulation (QSR), or Current Good Manufacturing Practice (cGMP) for Medical Devices. It outlines the requirements for the design, manufacture, packaging, labeling, storage, installation, and servicing of medical devices. It establishes the quality system requirements that manufacturers must follow to ensure their products’ safety and effectiveness.
Software OEM services
Although not software-specific, companies validating to FDA 21 CFR Part 820 through a qualification plan, will inherently need their chosen software solution to be implemented to this regulation. Integral elements are Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
OEMs can offer their end customers a service to facilitate meeting these qualification standards in relation to the systems they have supplied.
- Installation Qualification (IQ) - essential to confirm that the testing system and its software are installed correctly and align with specified requirements. This involves meticulous verification of hardware and software components, comprehensive documentation of installation procedures, and confirmation that the system is ready for operational use.
- Operational Qualification (OQ) - focuses on validating the operational functions of the testing system and its software. This involves testing features and functionalities under various conditions to guarantee consistent and accurate performance.
- Performance Qualification (PQ) - ensures that the testing software consistently produces accurate and reliable results within its intended environment. Execution of test cases, thorough analysis of results, and comprehensive documentation all contribute to confirming the software's overall performance.
The team works
The indispensability of software in industrial and medical product testing lies in its ability to guarantee not only accuracy but repeatability and reproducibility. By meeting stringent data security requirements and adhering to Installation Qualification, Operational Qualification, and Performance Qualification standards, testing software becomes the cornerstone of manufacturing excellence. As industries evolve, investing in robust testing software, which works hand-in-hand with its users, emerges as a strategic imperative, ensuring the delivery of high-quality and reliable products to their consumers.



