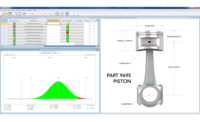
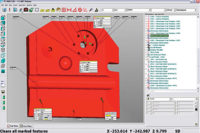
Automated SPC software can help organizations take their quality approaches from reactive to proactive, boosting efficiency and getting everyone on the same page.
Read on to learn how companies in transportation and defense, consumer goods and construction materials, food and beverage, and medical devices and pharmaceuticals companies can meet quality requirements, industry regulations and more
Transportation and defense
Manufacturers and contractors in transportation and defense fields can lower the cost of quality and reduce scrap with SPC software.
Because these companies are legally required to validate that their parts are made to customer specifications, they benefit from software that proves this.
They are also expected to regularly show that they’re meeting quality levels. Companies that machine, stamp, mold, weld, or otherwise use a precision process, must follow well-known quality measures and critical quality checks. They must make corrections when deviations or defects do occur. By storing data in a central system, transportation and defense manufacturers can easily monitor and continuously improve their quality programs. They can also easily export reports.
They can also use SPC software to:
- Routinely gather data from gages, CMMs and other metrology devices
- Train operators in minutes
- Recognize trends and prevent parts from being machined outside tolerances
- Rapidly produce professional quality reports
- Monitor performance by machine, cell, part number, and lot
Consumer goods and construction materials
Consumer goods and construction materials industries use SPC software to reliably produce high-quality products while constantly improving manufacturing productivity, which is key to quality and customer satisfaction. This is essential in such a competitive, high production volume industry.
Consumer goods and construction materials industries produce extrusion, fabrication, machining, assembly, and additional precision processes, which can benefit from standard dimensional checks and continuously observed test results, defects, and other factors.
SPC integrates with automated inspection equipment, helping consumer goods and construction manufacturers to boost customer satisfaction, product and process quality, and reduce warranty repair costs.
Food and beverage
SPC enables food and beverage companies to automate the gathering of weights, temperatures, and other product quality measures, and then regulate processes with real-time charts and alarms.
It helps food and beverage producers to identify and address process shifts in real-time and continuously monitor control parameters. They can use dashboard views, reports, and analysis tools to monitor activities and investigate issues, which helps them to noticeably improve performance and reduce variability.
Additionally, SPC software uniquely helps this industry to:
- Use alarms to alert supervisors of trends by SKU, line, fill head, and more
- Connect to scales, check weighers, vision systems, and metal detectors
- Support SQF and industry regulations
- Analyze, detect, and control the variability that causes overfill or underfill
Medical devices and pharmaceuticals
Manufacturers in the medical devices and pharmaceuticals industry can use SOC software to reduce product defects, lower manufacturing costs, and attain regulatory compliance. In such a regulated industry, manufacturers often struggle to make quality products at low costs without overburdening their workforces.
Companies in these fields must track hundreds of quality tests and maintain that data for a long time. Automated SPC can help them to manage a range of machining processes, such as plastic molding, chemical mixing, lab analysis, assembly and packaging.
It can also identify root causes of issues, removing another manual task for a staff that’s already busy. Lastly, it can uncover unknown process variations and provide timely feedback to trends and patterns that lead to defects. This reduces the inaccuracy.
With SPC, manufacturers and suppliers in the medical devices and pharmaceutical industries are able to:
- Improve product and process quality
- Meet FDA requirements
- Offer system-wide audit trails
- Prevent product defects and recalls