SPC quality control keeps medical and pharmaceutical manufacturing processes operating at their highest levels.
Because raw medical materials and parts are expensive, finding defects late in production can be costly. Such errors increase scrap and rework along with shipment delays.
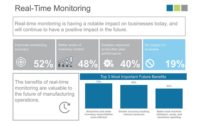
To prevent such defects ahead of time, quality managers can use SPC software to closely monitor processes at the earliest stages of production. This helps them gain visibility to automated, real-time production-floor data through alerts, monitoring, and analysis. In the long-term, it also helps them to enable a corrective and preventive action system before a problem develops, boost product quality and process efficiency.
Such software also automates data collection, inputting information into a single, centralized repository. Manufacturers can use the built-in database to create definitive traceability for every product and component. Spreading automation to incoming inspection and integrating with devices saves both time and labor.
The medical device industry faces a particularly high volume of scrap, as materials endure many metalworking phases to achieve the right dimensions and finish. Although manufacturers document these scrap and yield numbers, many do not track them over an extended period. The right SPC software can do this and can show why the numbers are so high. It can monitor scrap and trace it back to its root cause. Regulating processes based on this analysis can decrease the amount of waste, cut costs and boost profits.
As a result, this reduces the likelihood of rework and scrap, as well as minimize any production and shipment delays.
SPC software also helps medical and pharmaceutical manufacturers abide by industry regulations, which are stringent in this space.
For example, the U.S. Food and Drug Administration, European Union directives, the European Medicines Agency, and Health Canada all require medical and pharma manufacturers to comply with safety rules. Quality managers can use real-time documentation and reporting, product traceability, and validation software to do this. While easily meeting the challenging FDA 21 CFR Part 11 requirements. Some programs even have built-in checklist features to help companies easily comply with Hazard Analysis and Critical Control Points, Standard Operating Procedures, Critical Quality Attributes and Critical Process Parameters.
Here is a sampling of the ways SPC software can help medical and pharmaceutical manufacturers comply:
- It prepares quality managers for audits with reports to support internal or external auditing activities
- It helps prevent recalls
- It helps managers quickly respond to product recalls and counterfeit products
- It enables managers to easily and quickly validate software
- It boosts medical quality control