Lean approaches are beneficial for the development and maintenance of management systems compliant with ISO 9001, ISO 13485, ISO 14001 ISO 45001 standards and 21 CFR 820 FDA regulation. An excessive number of documents, over-detailed and over-decorated “Standard Operating Procedures” (SOPs) are ineffective and costly. On the other hand, well-written and organized procedures can lead an organization to a more efficient Lean management system. The article offers an effective tool to document and monitor the progress in the transition to a Lean ISO management system, and will be helpful to those organizations that struggle with over-documented management systems and those that are searching for assistance in optimizing their ISO procedures.
Problems
According to research by the Lean Enterprise Research Centre, 60% of production activities in a typical manufacturing operation are waste! Through my consulting and auditing career I have worked with hundreds of businesses. In various countries, in different industries, in companies large and small I keep on finding, again and again, the same problem: management systems are over documented. Very few companies, if any, apply Lean techniques to their management and documentation systems. This is bad news; the good news is that there is a simple and sophisticated solution.

A simple document format as shown here is much easier to use.
The solution is Lean
Let’s talk about how Lean approaches can eliminate waste and help you simplify your ISO 9001, ISO 13485, ISO 14001 or ISO 45001 management systems. The Lean approach originated in Japan. Originally, the concept was applied to manufacturing and production processes. The objective is simple: eliminate or minimize waste within the process without impacting the outcomes. To transition to the Lean state in documentation one has to identify and eliminate waste in procedures: those attributes and steps that do not contribute to the purpose of a procedure. In our research, we identified close to 30 typical elements of waste in procedures. The biggest “offenders” in a non-Lean management system are:
- An excessive number of documents
- Long and wordy titles and content
- Duplicate revision history and approval tables
- Use of excessive “decorative” graphics
- Use of passive voice
- Use of document numbers, often not justifiable
- Use of reference documents, responsibilities, and other sections that are frequently duplicated within the text of the procedure
Often these deficiencies make procedures wordy and long, difficult to read, navigate, understand and follow. Below are a few examples of the negative impacts of some waste categories.
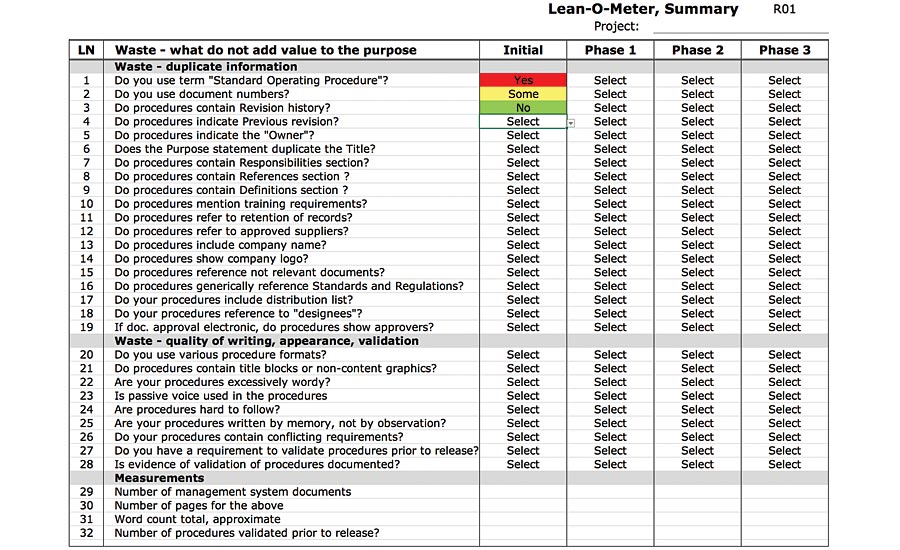
This tool helps to identify and document waste in procedures.
An excessive number of documents
Despite very limited standards requirements for documented information, organizations continue to generate unnecessary documents. Once I worked with a large multi-location company in which management system documentation presented an interesting case study.
The company comprised a home office, nine divisions with some nine to 10 locations for each division. The home office had created, among others, a management review policy and a management review procedure. The practices required each division to develop its own management review policy and management review procedure. All divisions simply copied the home office policy and the procedure, only replacing the name with the name of the division. By now, 20 procedures have been in place for the management review process. If you think this is too many or too funny, wait for the rest of the story.
The practices also required each location to develop its own management review policy and procedure, bringing the total number of management review procedures to some 210 documents, instead of just one for all locations! The same documentation structure with some 210 procedures was used for internal audit, corrective and preventive action, and other processes. Fortunately for the business, our discovery got the attention of the home office decision-makers who acknowledged waste in the system.
Too wordy, hard to follow
Let’s start with our notorious “Standard (or even Standing) Operating Procedure” (SOP). Decades ago, the military introduced this bureaucratic term and since then, by some unknown reason or just apathy, businesses small and large polluting their documentation systems with it. Can anybody tell me if there are “non-standard” operating procedures or “sitting” instead of “standing” operating procedures? If not, how do businesses benefit from using three words instead of one? What if from now on we call those standard operating procedures simply procedures? This will become our first step toward our Lean ISO documentation.
New or revised documents are not validated
Rarely organizations specify requirements for or perform validation of new or revised procedures. It always results in perpetual re-work of documents and waste of resources and time. One of our clients was drowning in a number of its document change records (DCRs). To investigate the problem, the company examined its DCRs for the previous three months. Sixty-seven percent of records indicated that the reason for the change was “corrected mistake.” The company revised its documentation change process to add a requirement for documented evidence of validation of a procedure, initial or revised. Six months later another sample of three months DCRs were examined. In the second round, the test showed that only 8% of changes were attributed to “corrected mistake.” Documentation management personnel indicated that changes became notably higher quality.
Excessive graphics
I seldom see procedures and instructions that start from the “meet”—the steps to perform the task. Often documents start from the revision history and a list of approvals. All this information is already in the document change system, so copying it in each procedure is duplication and waste! Honestly, how often did you pull a procedure to see what revision it is and who approved it? I am not sure about you, but I know definitely that I never wanted to see a procedure to find out its revision history or who approved them.
Let’s remember that the purpose of a procedure is to define steps to complete a particular process or a task. So, per the Lean method, anything that does not contribute to the purpose is waste!
Document numbers
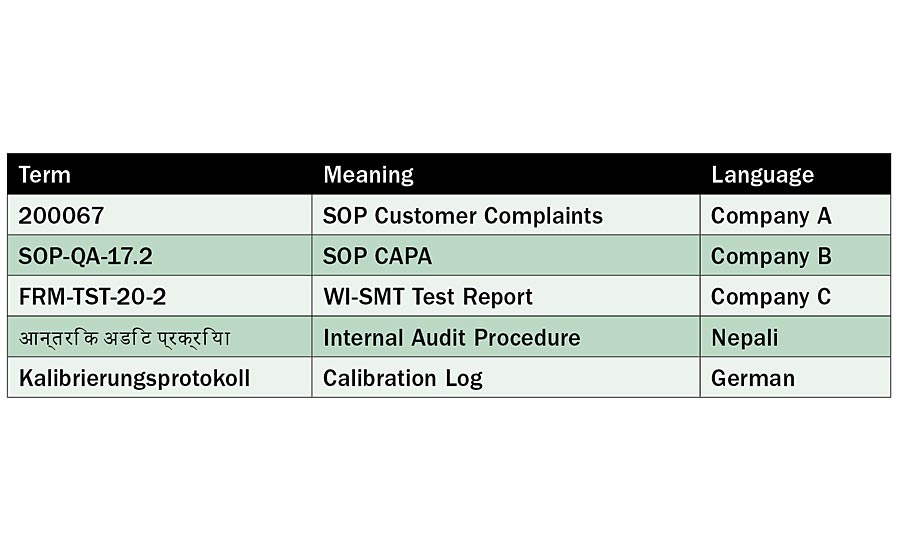
Can anybody say what words and numbers below mean?
200067; SOP-QA-17.2; FRM-TST-20-2; आन्तरिक अडिट प्रक्रिया; Kalibrierungsprotokoll
No? Sad, but understandable: we cannot translate these numbers or terms because we do not speak those languages! To learn what they mean, see the chart on the left.
Where am I going with this? Maybe you already guessed: document numbers in small systems are waste. Only a small number of employees in a company speaks and understand the Number language. If you have 100, 200 or even 500 documents, the title in your native language with a revision level is a sufficient identification to satisfy any standard, regulation or business needs. You may run out of unique titles and will need numbers if your system supports building submarines, complex construction projects or other large developments. Unless justified, numbers do not contribute to the purpose of documents. There is a simple test for Do-We-Need-Numbers:
- List your documents alphabetically
- Find duplicate titles
- If you see one or two—change the titles
- If there are many duplicate titles, and renaming is not practical—numbering is justified
Lean-O-Meter
At the beginning I indicated that our surveys of dozens of companies identified close to 30 waste categories littering procedures. We listed them in out Lean procedure test tool that we also call Lean-O-Meter. This tool helps to identify and document waste in procedures. It also provides a visual dashboard to manage and report progress in the lean management system improvement project.
On the case study example, we show how this tool works on our YouTube channel at https://www.youtube.com/watch?v=6qt50xqoBPM&t=22s.



