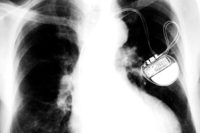
Medical devices can present unique challenges for manufacturers. Consequences for malfunctioning equipment can be dire. Because of this, medical device manufacturers must work hard to ensure that their products never fail.
“Medical devices are unique in the scrutiny to which their quality is measured and guaranteed,” says Landon Goldfarb, senior applications engineer, Instron. Because medical devices are regulated by the FDA, “both the amount of testing and the verification of results is held to a higher standard than other products,” he says.
Medical devices warrant relatively high levels of traceability in order to obtain, track and analyze data, says Goldfarb. All lab equipment, not just mechanical test equipment, is subject to these regulations.
“The software required to remain compliant with these regulations can be complex and needs to address all the nuances of the regulation while remaining user friendly to prevent user error,” he said. “It is sometimes such a hassle that companies choose to continue using paper trails to avoid meeting [certain regulations].”
So, how do organizations keep up with regulations, especially as they continue to change?
Anne Holland, founder and CEO of QA Consulting, credits industry newsletters, webinars, and industry meetings with helping her organization stay up to date. She also advises organizations to create ongoing internal processes to monitor changes and trends, “whether that’s an individual conducting research or creating and monitoring a tool such as a Google alert,” she says.
Additionally, management and staff must be aware of the impact of regulatory changes, she says, and should “consider the actions and timing required to comply.”
Goldfarb says Instron remains “plugged in” to the industry’s major testing bodies, including ASTM and ISO, and its staff even participate in these associations’ committees, helping to develop testing.
“By having a membership in these committees, we can better understand the challenges faced within the industry as it relates to FDA and other governmental regulation,” he says. And as voting members, “we can even be on the forefront of standard changes, modifying our products to better meet new standards and regulations.”

Analysis of fit and function of a defibrillator. Three-dimensional influences make 1D stacks insufficient. Source: 3DCS
Keeping up with regulatory changes is even a challenge for certification bodies, says Colm O’Rourke, industry engagement officer for the National Standards Authority of Ireland’s [NSAI] medical devices division. Joining both local and global working groups, such as TEAM NB, gives NSAI the opportunity to stay abreast of regulatory changes, he says.
Benjamin Reese, marketing manager of 3DCS.com, a company that develops dimensional engineering and quality data software and services, says the best way to keep up with regulations is to allow for variable inputs, and to create customizable outputs for devices.
Simulation software can help organizations to leverage a variety of different inputs, and to change those inputs as needed, he says.
“If new quality regulations are released, or if, after running an analysis, components do not meet the requirements, the model inputs can be quickly altered in order to meet the specified requirements,” he explains.
“With the promotion of Quality 4.0, these tools are now becoming affordable and interconnected in a way that provides visibility into product quality like never before, at a pace and speed that is constantly increasing,” he adds.
Manufacturers can also use software to customize outputs, which helps manufacturers create FDA-mandated compliance documents without burdening production, he adds.
The increasing focus on quality and regulatory at an international level has heightened the awareness of early implementation of quality processes, says O’Rourke.
“The depth with which certification bodies now go into in terms of design history means that companies need to be conscious of these requirements at a very early stage,” he says.
Staying current with continually changing government and third-party regulations is tough, Holland says, but manufacturers can achieve consistent quality by aligning user requirements, design documentation, supply chain concerns, inspection and test results, identification and more from the get-go.
“The key to ensuring quality is internal alignment throughout the lifecycle of a medical device, from the original device concept to post-market activities,” Holland says. “This can be a complex process but it’s an investment that will lead to consistent quality and is just as important as regulatory compliance.”
To properly develop and market a device—be it a software application or a high-risk implant, Holland says, organizations must integrate “the overarching risk-management and feedback loop,” which must be integrated within a controlled quality management system. This gives medical device manufacturers “both the stability and flexibility to stand out and successfully compete in the industry,” she says.
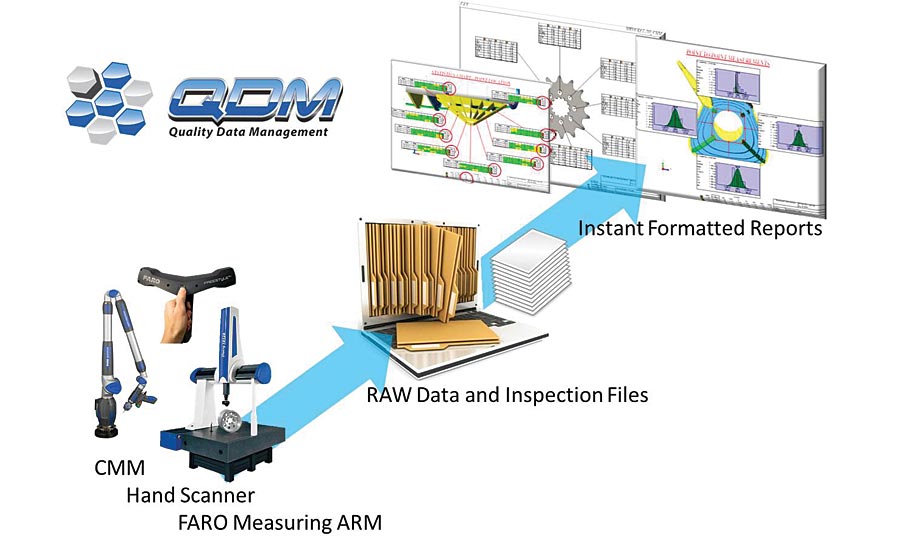
Automated SPC systems allow for faster compliance and quality reporting. Source: 3DCS
Industry 4.0 and value
As manufacturers contend with rising costs, increasing regulation, decreasing product life cycles, and lengthening supply chains, medical devices can be tough to make affordably, says Reese.
“Whereas some industries can afford to lag behind the cutting edge, it is important for medical device manufacturers to stay abreast of new developments in order to constantly battle [this],” he says.
Fortunately, the cultural and collaborative gains made by Industry 4.0, or the digitalization of quality, management systems and compliance, have made it possible for manufacturers to afford tools that “can support their visions and provide the answers needed to make important quality decisions,” Reese says.
“With the promotion of Quality 4.0, these tools are now becoming affordable and interconnected in a way that provides visibility into product quality like never before, at a pace and speed that is constantly increasing,” he adds.
Such tools include three-dimensional stack tools, which help organizations manage tolerances. While they may come with a learning curve, Reese says, they help manufacturers to catch significantly more issues in production, account for variation and tolerances from the entire assembly and more, compared to their counterparts. These tools provide better insight into products and their components, which enables medical device manufacturers to better predict how their products will behave and adjust designs and processes accordingly.
3-D printing takes hold
Historically, 3-D printing has been predominantly used for prototype development, but has recently been adopted for routine component production, says O’Rourke.
While this technology will add “an extra layer of complexity” quality control processes, it “supports the development of more customized solutions for patients and therefore should be embraced from a certification point of view,” he said.
Reese commends this customization and its ability to help patients, but notes that its rise warrants increased validation, “especially as 3-D printers begin to become faster, more efficient, and most importantly, cheaper,” he says.
“Once manufacturers are able to create large production banks of 3-D printers, there will be a greater need to optimize designs to account for variation, cooling, material, and stresses that will allow for mass production,” he adds.
Globalization’s role
As outsourcing of medical device manufacturing and testing continues to grow, device manufacturers and contract suppliers must collaborate, Holland says.
As technology, materials and design capabilities, and even expectations for both quality and regulatory compliance quickly change, design firms, software developers, manufacturers and test facilities—as well as sterilization and packaging experts—should work to generate designs, manufacturing and test methods concurrently, she says.
Globalization of component production has affected medical device manufacturing just as it has impacted other production sectors, says Reese. “Like manufacturing in other industries, the growing globalization of product sourcing and the globalization of supply chains has created more competition in the market,” he says. This in turns reduces product life cycles and makes it more difficult for engineers and designers to validate and test products.
It also presents complications for international suppliers who adhere to different quality standards.
“These factors have pushed medical device manufacturers to look for systems and tools to help monitor suppliers,” Reese say, “and to streamline their validation and design processes.”