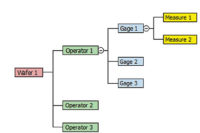
Gage management provides manufacturers with data to ascertain the quality of their parts.
Such adjustments include gage repeatability and reproducibility (GR&R) studies and regular calibrations, which is a means to verify that manufacturers’ gages are accurate and in good condition. Gage studies determine if the gage is appropriate for the measurement needed.
Gage management is central to quality efforts because it deals directly with measurement—a foundational requirement for quality, says Alex Zaks, president, Altegra.
“Can we trust the measurements we obtain in process control and assessing product quality? If we do not have quality of measurements, then we do not have measurement of quality,” he says.
Gage management encompasses four main areas, each addressing an important aspect of a comprehensive approach to measurement quality, Zaks says. They include:
- Inventory management, which indicates if the right gages are available.
- Calibration management, which reveals when instruments are due for calibration, when they were calibrated, and the calibration results.
- Usage history, which allows businesses to track potentially defective products if a bad gage was used to take measurements, and
- Measurement system analysis, specifically GR&R studies, which enable organizations to assess the measurement system’s fitness for use, which results from the performance of the instrument, appraiser’s skill, and the product feature being measured.
GR&R studies’ role in gage management
GR&R studies determine if a manufacturer’s measurement system is capable of providing readings that can be used in deciding part acceptability. Ultimately, it will indicate how repeatable and reproducible a gage and measuring method is. Results from GR&R studies allow businesses to identify where variations in measurement processes can be improved.
GR&R studies prove repeatability when one operator measures against a known value over several instances and determines the variation from the measurements. They prove reproducibility when multiple operators repeatedly measure against a known value.
Gage management software also allows non-specialists to perform complex analysis.
“The idea is to make sure that the instrument, in the hands of a trained operator [is] accurate and repeatable,” says Robert F. Brown, senior product manager, manufacturing at Epicor. GR&R studies are designed to ensure that poorly performing instruments and/or untrained operators are not introducing variability into the measuring process, he says.
GR&R studies are critical, Zaks says, because they can prevent gage misapplication and the subsequent false negatives or false positives in assessing product quality. They also go a long way in organizations that don’t have staff expertise in this area.
“For companies with small quality staff, GR&R capabilities in their gage management software not only automate the GR&R process but provide built-in expertise,” he says. “It’s much easier to train someone in how to use the software to perform a GR&R than to train someone in understanding the underlying statistical concepts and the computations required to carry out the GR&R studies.”
Despite their value, experts say that the manufacturing field has not universally embraced GR&R studies.
“GR&R is very well established in some companies but in a very rudimentary state at others,” Zaks says. “It is often driven by the nature of the products manufactured and the quality system requirements imposed by the company’s customers. The use of GR&Rs range from being a routine, must-have part of the quality system performed in dedicated software to ad hoc requests done in a spreadsheet.”
Jack Gaughan, vice president, sales at Edmunds Gages, says that the results of GR&R testing is the most overlooked gage management tool that high volume, variable gaging holders ignore.
“Most of our customers hold us to a 10% or less GR&R result when buying new gaging. I get that—they want the most for their money,” Gaughan says.
“However, that’s often the last time a GR&R is performed on said gage, until there is trouble—either with their customers’ acceptance of their output, or ambiguous readings by other methods of measurement,” he says.
Gaughan says that Edmunds Gages “always recommends” that customers hang on to their 10-piece GR&R samples and test the gages at least twice a year.
“It’s the first thing our service technicians will do when arriving on a gage service call,” he says.
“A degrading GR&R often tells more, in terms of wear, contamination, or environmental effects on a gage, than any observation or visual inspection of a gage will ever tell,” he adds.
Strong gage management and tracking impacts a manufacturer’s ability to grow, experts say.
If gages are not maintained properly, manufacturers are likely to face an increase in measurement variability, which erodes customer confidence. Through consistent delivery of high-quality products, organizations can boost their business.
“[It] is important in high compliance and reliability manufacturing since without [it], you will not be able to pass customer audits of quality systems,” says Brown.
Solid gage management promotes productivity and an organization’s ability to handle complexity, which ultimately strengthens the business, Zaks says.
Gage management software can help a manufacturer to manage greater volume of calibration activities without necessarily adding staff. Without gage management software, an organization’s internal calibration lab can become overwhelmed with work, he says.
“Gage management software enables quality professionals to accomplish the same tasks in less time and with less effort, thus leaving more time and more intellectual energy for new project,” he says. “As the manufacturer grows, the number of measuring instruments grows, as well.”
The evolution of gage management
Paper-based gage management, once the industry standard, is essentially nonexistent today, Zaks says.
“Even the smallest operations with a few dozen gages use some form of computer-based calibration tracking,” he says.
Businesses are currently migrating their records from spreadsheets and homegrown systems to commercial gage management software and are upgrading older products to the new generation of software, he says.
Companies that move away from spreadsheets to obtain the productivity and the ease of use offered by specialized software see reduced training time and human error,
he says.
Organizations that use such homegrown systems often rely on the person who spearheaded the system to maintain it—which is problematic if that person leaves the business or grows out of the role, Zaks says. And as administrative software updates over time, some original systems might not keep paces with those updates and can become unusable.
“Eventually, the once-effective solution becomes a business risk,” he says. “And none of the solutions developed in-house can keep up with the evolution of commercial gage management software which is propelled by market competition.”
Thus, companies using the latest commercial software gain a competitive edge including such capabilities as business intelligence and performance analytics, Zaks says.
Automated gage management software that incorporates quality data is key, says Christine Hansen, senior product marketing manager at Epicor.
“As we businesses continue to employ more devices and sensors to automate quality processes, we naturally are collecting more quality data as a result,” Hansen says. “Managing this data to more proactively predict failure, even in gages and other devices used in the function of validation is critical to minimizing business disruption and optimizing business productivity.”
Gage management software also allows non-specialists to perform complex analysis, Hansen says.
“We are seeing continued expansion in not only management of gages for accuracy but [also a] continued move towards digital devices and placement of devices at the point of manufacture,” she explains. “A great example is Epicor customer Perceptron, who develops, produces and sells a comprehensive range of automated industrial metrology products and solutions to manufacturing organizations for dimensional gaging, dimensional inspection and 3D scanning. Their devices are used in automotive, aerospace and other manufacturing companies.” Q