Lean approaches are helpful for the development and maintenance of documentation compliant with ISO Management System Standards (MSS). These standards include ISO 9001, ISO 13485, ISO 14001, ISO 45001, and many others. You can also apply these tactics to the management systems to support 21 CFR 820 FDA regulation. The article offers an effective technique to optimize management system documentation structure. It will be helpful to those organizations that struggle with over-documented systems and those searching for ideas to simplify their ISO management system documentation.
Problems
According to the Lean Enterprise Research Centre research, 60% of production activities in a typical manufacturing operation are waste! One may find the same, if not more, in typical ISO management system documents. Typical deficiencies in procedures include:
- Documents are too wordy and too long
- Poorly writte
- Content is complicated
- There are duplications
- Documents are outdated
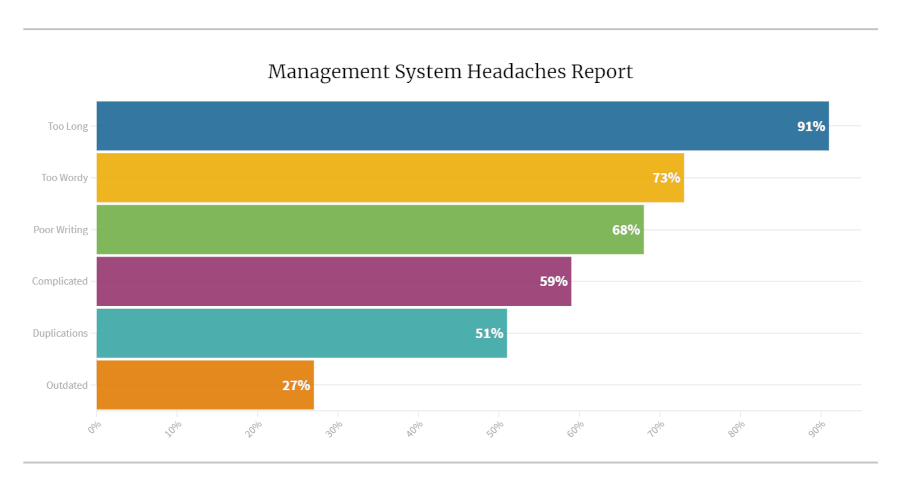
Our recent survey asked eighty-three management and supervisory personnel in five organizations to assess their procedures against this list. The results were thought-provoking.
 (Click on image to enlarge.)
(Click on image to enlarge.)
Almost all the folks interviewed thought their procedures were too long. Two-thirds felt that documents were too wordy and poorly written. Yet, very many companies continue burying themselves in unnecessary and hard-to-follow procedures.
The solution is Lean.
 © 2020 Lean ISO Management Systems (Click on image to enlarge.)
© 2020 Lean ISO Management Systems (Click on image to enlarge.)
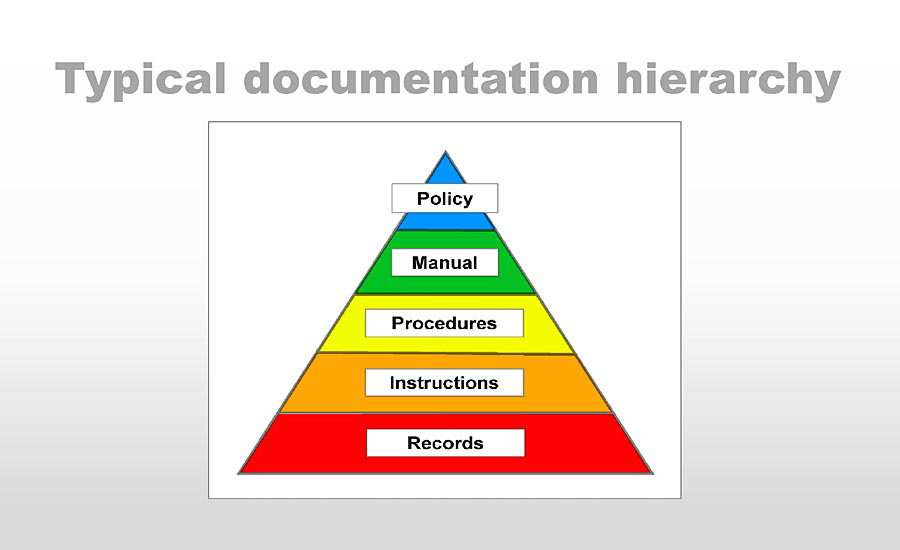
Businesses typically document their management system structure as a pyramid. Most of the manuals I have seen contained a section called “Documentation Structure.” All these chapters were alike as twins and showed the same hierarchy: the top-level is the manual or a policy; the second level is SOPs or procedures, then instructions, then records. Some structures had a manual on the top, some the policy. Let’s put aside that a manual cannot be the top document because the policy defines which standard a company will comply with. Only then, the organization creates a manual to support the policy.
This pyramid is an excellent place to start our optimization example, but before we continue, a quick reminder. Since the introduction of Annex SL, no management system standards require quality or business or any other manuals. I keep on referring to manuals only because most companies still maintain and use them. One of my YouTube videos (the link is below) showed how one could develop a Lean Business Manual. That manual ended up being a three-page document instead of the typical thirty, forty pages.
 © 2020 Lean ISO Management Systems (Click on image to enlarge.)
© 2020 Lean ISO Management Systems (Click on image to enlarge.)
To demonstrate how we can Lean up a documentation structure, let’s take any clause of the ISO 9001 2015 standard that requires records. For this example, I picked element 8.4.3: Information for external providers. This clause requires that “The organization shall communicate to external providers its requirements for … products and services…” In a typical documentation structure, the first layer is the manual. As a rule, the manual simply rephrases the standard, changing “the organization shall” to “we will.” The next step is the Purchasing procedure. Typically, the procedure mimics the manual with additional details, such as References, Responsibilities, and other sections. Finally, what in fact defines your company’s needs and addresses the standard’s requirements is the Purchase Order itself. Let’s see how we can avoid all these duplications and get out of this clutter.
 © 2020 Lean ISO Management Systems (Click on image to enlarge.)
© 2020 Lean ISO Management Systems (Click on image to enlarge.)
Since we eliminated the wordy manual, we are one step ahead in the game. The copy of the standard in the manual is gone! Now our structure looks much Leaner—only the procedure and the record are left. Our record is a purchase order that communicates to the supplier our needs. Let’s keep in mind that records often contain sufficient responses to the requirements of the standard and the needs of your business. If not, simple enhancements to the template record may eliminate the need for a procedure.
 © 2020 Lean ISO Management Systems (Click on image to enlarge.)
© 2020 Lean ISO Management Systems (Click on image to enlarge.)
So, if we can leave without the procedure, our documentation structure for this element of the standard may look something like this. It is much more straightforward and much Leaner.
To summarize:
- Eliminate the manual or use Lean Manual, if you choose to have one,
- Ensure that appropriate records contain sufficient details to address the needs of the company and requirements of the standard, and
- Consider eliminating the corresponding procedure.
Mark Kaganov is a principal consultant with Lean ISO Management Systems. The company provides consulting and training on developing and implementing Lean management systems and documentation compliant with ISO 9001, ISO 13485, ISO 14001 standards, and 21 CFR 820 FDA regulation. For more information, visit LeanISOManagementSystems.com. You also may explore how to create a Lean Business Manual on the YouTube Channel at https://www.youtube.com/watch?v=sbr52i5XSbY&t=2s


